-40%
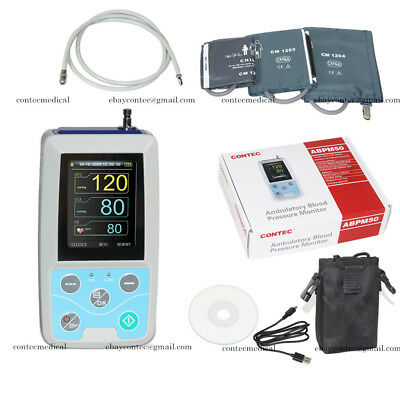
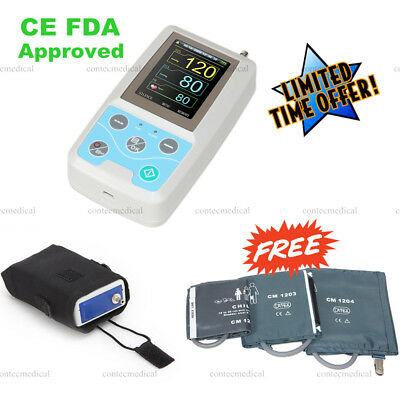
USA 24h Arm NIBP Monitor Care Ambulatory Blood Pressure Monitor ABPM50+3 Cuffs
$ 73.39
- Description
- Size Guide
Description
USA 24h Arm NIBP Monitor Care Ambulatory Blood Pressure Monitor ABPM50+3 CuffsIntroduction
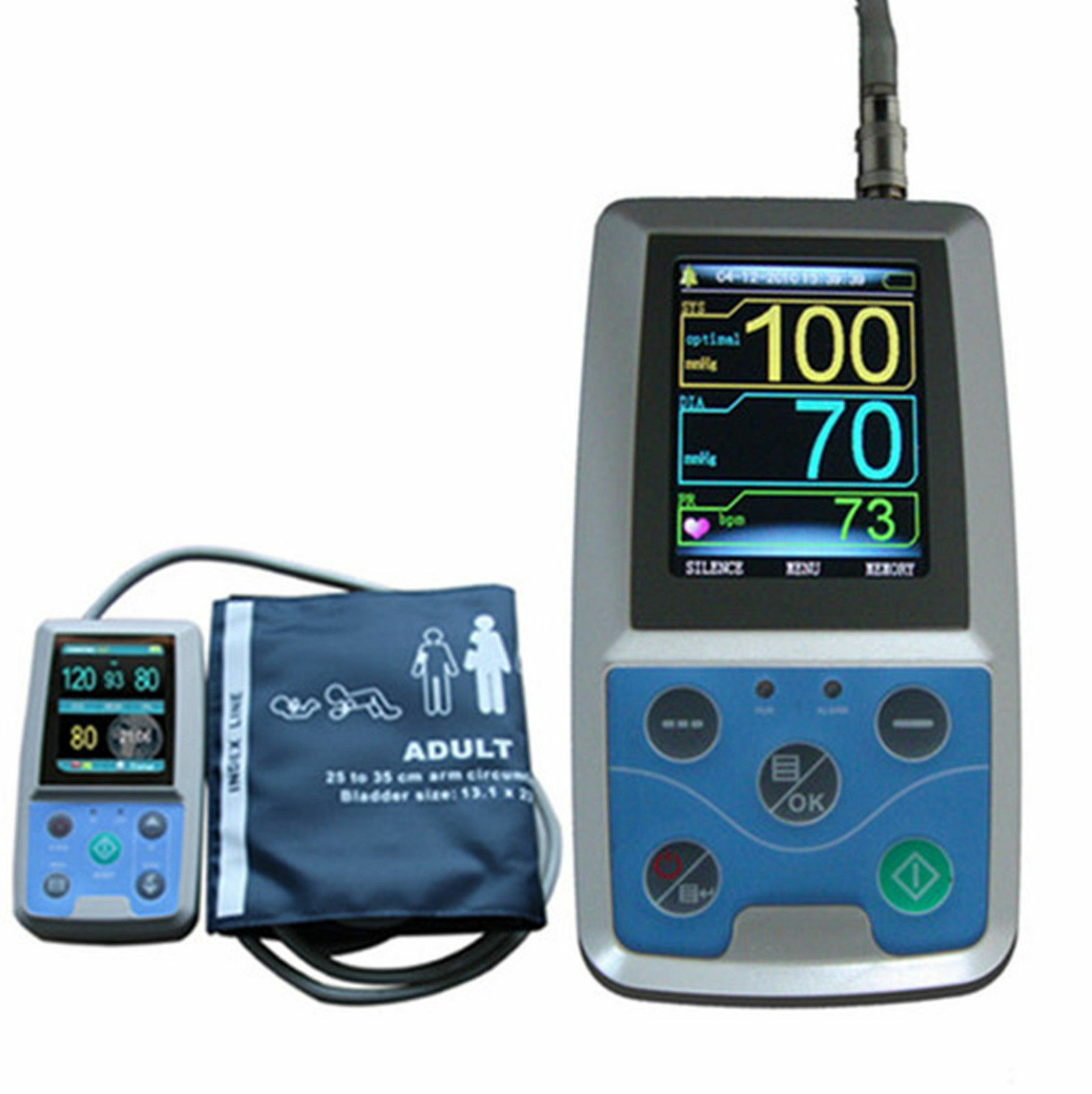
ABPM50 is a handhold ambulatory blood pressure monitor, which is designed according to oscillography theory. The device could monitor human body blood pressure up to 24 hours continuously and dynamically, providing accurate basis for the diagnosis. It is applicable for using in hospital, clinic and other medical institutions.
Features
Product features:
1)Compact and portable, user-friend interface, easy to use
2)Patient range: adult, pediatric, neonate
3)24 hours ambulatory NIBP monitoring function, up to 350 groups of ambulatory NIBP data can be recorded for once.
4)Perfect combination of automatic and manual measurement method, up to 300 groups of data can be recorded for once by manual measure.
5)High-definition color TFT display, strong visibility
6)By data review interface such as "data list","trend graph","big font", NIBP data is clear at a glance
7)Display of low power prompt, alarm, error message and time
8)Supply two kinds of unit: mmHg / kPa
9)Display interface can be switched between Chinese and English
10)Parameter alarm dispose function is optional
11)Communicate with PC, PC software can achieve data review, measured results analysis, view of trend graph, reports printing and other functions
Software features:
1)Connect to the device by USB interface.
2)Download NIBP measure result from the terminal device.
3)Display of scoop-shape trend graph, filling-type trend graph, histogram, pie chart, correlation line graph.
4)Edit every piece of NIBP data, and add annotation to it.
5)Edit basic information, doctor's advice, NIBP status instruction, current medicine-taken information, etc.
6)Support report printing and print preview.
Performance
NIBP:
Measure Method: Oscillometry
Measure Mode: The upper arm measure
Automatic Measure Interval: 15, 30, 60, 120, 240 minutes
Measure range: Pressure:0kPa (0mmHg)~38.67kPa (290mmHg)
Resolution: 1mmHg
Accuracy: ±3mmHg
Alarm parameter: SYS, DIA
Inflation: automatic inflation by force pump
Deflation: automatic multistep deflation
PR:
Measure range: 40bpm~240bpm
Resolution:1bpm
Safety:
Power supply: DC 3V (2×1.5V AA alkaline dry battery)
Safety type: internally powered device, type BF applied part with defibrillation protection
Accessories
Cuff for child 1pc
Cuff for adult 1pc
Cuff for large adult 1pc
CD (PC software) 1pc
User manual 1pc
USB data line 1pc
Pack 1pc
Physical characteristic
Dimension: 128mm(L) ×69mm(W) × 36mm(H) (without packaging)
Weight: <300 g (with battery)
Operational environment
Temperature: 5 ˚C~40 ˚C
Relative humidity: 15%~80%
Atmospheric pressure: 700hPa~1060hPa
Specific EMC, climate, mechanical environment: Do not use a mobile phone in the vicinity of the device, for the strong radiation field produced by mobile phone would interfere with the normal function of use.
Storage environment:
Temperature: -20˚C ~ +55 ˚C
Relative humidity: ≤95 %
Specific EMC, climate, mechanical environment: The device after packaged should be stored in a environment of -20℃~+55℃, relative humidity no more than 95%, and well-ventilated room with no corrosive gas. Strong shock, vibration and snow and rain should be avoided during transportation
Buy Safe Product!!
■
The following FDA Disclaimer is required for all eBay listing in
Healthcare category and is included for REFERENCE:
■
The sale of this item may be subject to regulation by the U.S.
Food and Drug Administration and state and local regulatory agencies.
■
If the item is subject to FDA regulation,We will verify your status
as an authorized purchaser of this item before shipping of the item.
■
The
model
is registered on the Australian Register of Therapeutic
Goods (ARTG)
with the code 197923,
and certified by FDA
510K Approved of America and CE,TUV of Europe
About Us
Contec Medical Systems Co., Ltd. (hereinafter referred to as CONTEC) focusing on research, manufacture and distribution of medical instruments, was founded in 1996 as a high-tech company. CONTEC locates in Economic & Technical Development Zone in Qinhuangdao covered an area of 125 acres and building area of over 100000 square meter, which is one of the largest bases for R & D and production of medical devices in China.
Contec hopes to cooperate with international companies to supply more innovative design and advanced
technology products
We sincerely welcome you to become one of our global partners.
We are looking forward to establishing a successful business relationship with you.
Contact Us
WhatApp:
+86 17749297380
:qiuyue0913(a)outlook.com
Only English user guide, if you need any other language, please contact us!